Chlorine Manufacturing and Production
Chlorine is an important chemical in everyday life and an important building block for the chemical and pharmaceutical industries. It is produced by electrolysis of brine together with lye and hydrogen.
Around 55% of European chemical production depends on this process and many chemicals, plastics and pharmaceuticals rely on chlorine in their manufacture.
Table salt is a key ingredient
One of the most common natural sources of chlorine is sodium chloride (NaCl), also known as table salt. Sodium chloride is common in ocean water and is also found in concentrated mineral deposits left over from ancient oceans that have dried up over long periods of time.
How is chlorine made from this common compound?
When sodium chloride is dissolved in water, a brine is created. Electricity is then applied to that brine to make chlorine gas (Cl2). This creates two other substances: caustic soda (usually in the form of sodium hydroxide [NaOH) and hydrogen (H2).
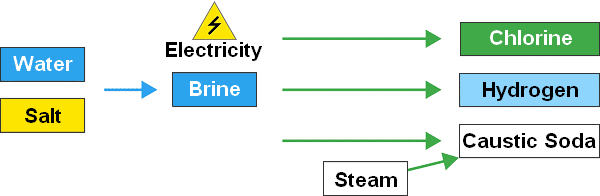
How is chlorine made?
After the salt is extracted, it must be transported to an industrial chlorine production facility, also called a chlor-alkali plant. The salt may be dissolved in water to create a brine solution and then transported by pipeline to the plant. Otherwise, dry salt is transported by rail or barge to a plant where it is dissolved in water to create brine.
However, the brine must be purified to remove possible contaminants, such as magnesium, calcium and iron that can interfere with the electrolysis process described below. To do this, the brine is filtered through ion exchange membranes that allow only sodium and chloride ions, along with water, to pass through the membrane.
The chemical reaction
The chlorine production process, which involves the industrial preparation and production of chlorine, sodium hydroxide and hydrogen, involves a common chemical reaction known as electrolysis. This reaction occurs when electricity is applied to brine. The electricity rearranges the elements in the brine.
Through two electrodes in the cell, the anode and cathode, electricity is applied to the solution. The anode has a positive charge, the cathode has a negative charge, and electrons flow from the anode to the cathode. The electrodes are immersed in the liquid brine. Between the electrodes is a selectively permeable membrane that allows positive ions to pass through - hydrogen. The selectivity of the membrane keeps the end products physically separated.
The positive charge of the anode attracts the negatively charged chloride ions. Upon contact with the anode, the chloride ions lose two electrons (oxidation). Immediately chlorine binds covalently to itself to make the following Cl2 gas that is removed from the electrolytic cell.
The negative charge of the cathode attracts the positively charged sodium and hydrogen ions (often called protons). Hydrogen ions and hydroxide ions are present due to the natural equilibrium state of water. At the cathode, the positive hydrogen ions gain electrons (reduction) and immediately bind covalently with themselves to form the following H2gas that is removed from the electrolytic cell.
At this point, there is an accumulation of negative hydroxide ions that bind with positive sodium ions to form sodium hydroxide in solution. The sodium hydroxide solution is then removed from the electrolytic cell.
Purification of end products
The electrolysis of brine does not fully convert the salt water into the end products. Depending on the specific conditions of the electrolytic cell, different concentrations of oxygen, salt and water are present in the end products.
Chlorine gas is contaminated with oxygen that needs to be removed. By cooling the gas, the chlorine turns into a liquid, while the oxygen remains a gas, allowing it to be separated. This process is called liquefaction.
Sodium hydroxide often contains salt residues in solution. The mixed sodium hydroxide and brine solution is heated to evaporate water. This makes the solution more concentrated and the salt precipitates out of the solution and is recovered to make more brine. The sodium hydroxide becomes up to 50% concentrated.
Reference(s) .. www.chlorine.org
Annual production of chlorine
| World | 65 million tonnes (1) |
| U.S. | 11 million tonnes (1) |
| Europe | 10 million tonnes (2) |
Data from:
(1) 2018 Elements of the Business of Chemistry, American Chemical Council.
(2) In 2017 Chlor-Alkali Review 2017/2018 Euro Chlor, 2018.
Sometimes the salt used is not sodium chloride, but potassium chloride (KCl). If KCl is used, the resulting products are chlorine, potassium hydroxide (KOH) and hydrogen gas.
Related Post(s)

When zinc or aluminum turns white, many people don't really know what causes this to happen. This article tries to explain the phenomenon...