Pickling and Passivation
What is Pickling and Passivation?
Pickling and Passivation are chemical treatments applied to the surface of stainless steel to remove contaminants and assist the formation of a continuous chromium-oxide, passive film. Pickling and passivation are both acid treatments and neither will remove grease or oil. If the fabrication is dirty, it may be necessary to use a detergent or alkaline clean before pickling or passivation.
Pickling
When steel is heated by welding or other means to the extent that a heat tint or oxide scale layer is visible, the layer below that has been depleted of chromium, thereby making the steel less resistant to corrosion. Pickling steel is the process of applying an acid solution to remove heat affected zones along with the underlying chromium reduced layer from the stainless steel. Pickled steel is free from surface carbon steel contamination and embedded iron particles. It typically leaves a dull, matte grey finish. Simply stated, pickling removes the heat affected layer of stainless steel and prepares the surface for passivation.
Passivation
Passivation is a process that is separate from pickling, which can be performed on its own or after pickling. Unlike pickling, the passivation process does not remove any metal. Instead, the surface of the stainless steel is treated with an oxidizing acid, to dissolve carbon steel, sulfide inclusions and remove iron and other surface contaminants from the stainless. The acid further works to promote the formation of the chromium-rich passive film, which imparts the corrosion resistance quality. Whereas pickled steel will appear dull or etched, passivation that is done correctly does not affect the metal's appearance.
Why Pickling and Passivation?
Many people are unaware of the fact that stainless steels are often in a natural passive state. However, whenever the steel is manipulated or fabricated in any way, such as when it is welded, the natural chromium oxide film on the metal surface is tarnished. As a result of that, the corrosion resistance of the stainless steel weakens following welding. To restore the chromium oxide film on the surface of the metal, one needs to passivate the stainless steel. As a matter of fact, one of the main reasons why stainless steels need to be passivated is because of welding. As a metal is welded, it loses its free iron from the alloy and the overall structure of the metal surface is transformed considerably. In the heat affected zone of the weld, the chrome to iron ratio is significantly decreased. The iron that is free flowing on the metal surface of the stainless steel can facilitate corrosion/roughing.
 Before Pickling
Before Pickling
 After Pickling and Passivation
After Pickling and Passivation
Which is the best passivation method available?
Passivation and Acid Passivation Treatment
It’s a well-known fact that stainless steel is corrosion resistant. What is less known is that a passive, chromium rich, oxide film that builds up naturally on the surface of the steel is responsible for the corrosion resistance. The formation of this passive layer is called passivation.
Passivation is a natural occurrence. However, it is possible to augment passivation by employing certain industrial techniques. These techniques involve oxidising acid treatments. It can be said that an acid treatment is the best method of passivating steel.
Ambient Temperature and High Temperature Corrosion Resistance
A simple oxide film or scale can be formed on the surface of the steel by applying heat. The same cannot be said for a passive layer. When heat is applied on the steel, the natural passive layer becomes thicker. In addition to changing volume, it changes colour as it goes from heat tint hues to a grey oxide scale. As a result of these visible oxide layers, the ambient temperature corrosion resistance of the steel is decreased.
Furnace parts are examples of stainless steel components that are tailor made to function in really high temperatures. In order to stay protected from high temperature oxidation, they are layered with these thicker, durable, oxide scale coatings.
On the other hand, in components that are made to work in ambient temperature environments, a thin transparent “passive layer” is enough to serve the purpose of corrosion resistance.
Requisites for Acid Passivation Treatment
Powerful oxidising conditions can be used to boost the formation of the chromium rich oxide passive layer on the surface of stainless steel. Nitric acid is the most suitable solvent for this oxidising acid treatment. Its availability, convenience and effectiveness makes it a widely used solvent in industrial stainless steel passivation treatments. Other more weak oxidising acids such as citric acid can also amplify passivation.
Steel components and fabrications that are derived from manufacturing mills and reputable stockholders are known to be fully passive. However, there are exceptions in each case. For instance, machined parts that have a complicated design and delicate structure may be in need of passivation treatments.
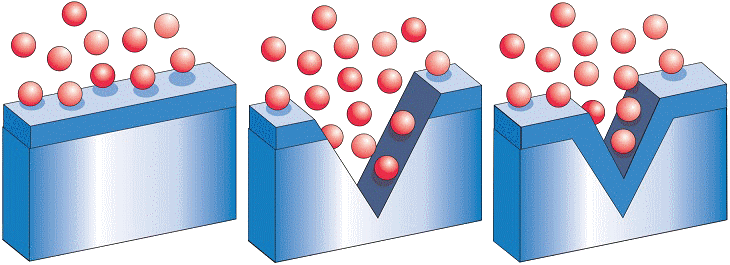
Stainless steel has a unique feature: It is self healing. Due to the alloying elements in the stainless steel, a thin, transparent 'passive layer' is formed on the surface.
Even if the stainless steel surface is scratched or damaged otherwise, this passive layer, which is only a few atoms thick, instantaneously reforms under the influence of oxygen from air or water.
This explains why stainless steel does not require any coating or other corrosion protection to remain bright and shiny even after decades of use.
Codes and Standards for Pickling and Passivation
- ASTM A380 Standard Practice For Cleaning, Descaling, And Passivation Of Stainless Steel Parts, Equipment, And Systems
- ASTM A967 Standard Specification For Chemical Passivation Treatments For Stainless Steel Parts
- ISO 16048 specifies the methods most often used for passivation of corrosion-resistant stainless steel fasteners
- BS EN 2516 Passivation Of Corrosion Resisting Steels And Decontamination Of Nickel Base Alloys
- AMS 2700 This specification defines the engineering requirements for a process to assure removal of free iron or other less noble contaminants from the surfaces of corrosion resistant steel parts.