Petroleum Refining Processes
A simple guide to Oil Refining
We all know that petroleum fuels and lubricants come from crude oil. What many people do not realise is that crude oil is also the starting point for many diverse products such as clothes, medical equipment, electronics, vitamin capsules and tyres. Whether on land or under the ocean, crude oil comes from deep underground where the remains of plants and animals from millions of years ago have been heated and pressurised over time.
Generally blackish in color, crude oil has a characteristic odour that comes from the presence of small quantities of chemical compounds containing sulphur and nitrogen. There are many different types of crude oil.
Each type has a specific composition that is determined by the original decomposed source materials as well as the properties of the surrounding soil or rock formations. It can be light or heavy, referring to density, and sweet or sour, referring to its sulphur content. However, in its raw state, crude oil is of little use. It must be refined to make it into useable products. Depending on the type of crude oil, it is treated via different refing processes to turn it into fuels, lubricating oils, waxes, chemicals, plastics and many other products used everyday in modern society.
The Refining Process
Once discovered, drilled and brought to the earth's surface, crude oil is transported to a refinery by pipeline, ship or both. At the refinery, it is treated and converted into consumer and industrial products. Three major refinery processes change crude oil into finished products..
• Separation
• Conversion
• Purification
Separation
The first step is to separate the crude oil into its naturally occurring components. This is known as separation and is accomplished by applying heat through a process called "distillation".
Separation is performed in a series of distillation towers, with the bottom product from each tower feeding the next. A furnace in front of each distillation tower heats and partly vapourises the feed stream. The vapour and liquid mixture is then fed into the bottom section of the tower. The feed section is the hottest point in the distillation tower and can reach as much as 400 degrees Celsius.
Components that are still liquid at this elevated temperature become the tower's bottom product. Components that are in vapour form rise up the tower through a series of distillation stages. The temperature decreases as the vapours rise through the tower and the components condense.
The "yield" from a distillation tower refers to the relative percentage of each of the separated components, known as product streams. This will vary the characteristics of the crude oil being processed. Because a liquid's boiling point decreases at lower pressures, the final distillation steps are performed in a vacuum to maximise liquid recovery. Products from the distillation tower range from gases at the top to very heavy, viscous liquids at the bottom. In all cases, these product streams are still considered "unfinished" and require further processing to become useful products.
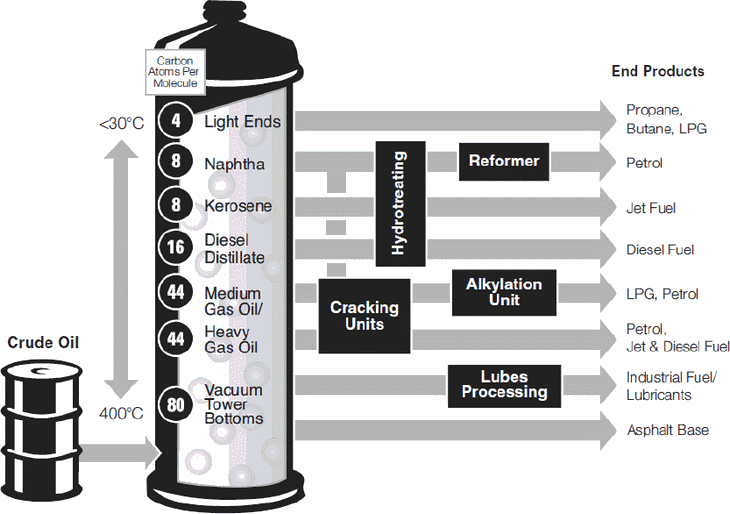
Just as water goes from liquid to vapour at approximately 100°C, each type of hydrocarbon changes from liquid to vapour within a specific temperature range. In general, the more carbons in a molecule, the higher its boiling point. This allows for separation within the distilling process.
Light products (light ends).. are further separated into propane, normal butane and isobutane (this stream is often referred to as Liquefied Petroleum Gas (LPG) and is sold as a cooking and heating fuel or as auto LPG for cars) and noncondensable gases (mostly hydrogen, methane and ethane) that are subsequently treated to remove trace impurities and are often used as fuel within the refinery;
"Naphtha".. could be blended into petrol, but is more likely sent to a Catalytic Reforming unit for octane improvement;
"Kerosene".. generally treated and used as jet fuel.
"Heavier distillate streams".. are also treated and blended into finished diesel fuel or home heating oil or are further processed in conversion units such as Fluidised Catalytic Cracking (FCC) and Hydrocracking. The routing of these streams will vary as product demand changes to either maximise diesel production or petrol production;
"Gas oil".. is routed to either FCC or Hydrocracking to be converted into higher value petrol and diesel;
"Vacuum tower bottoms (VTB)".. the final bottom product of distillation, which maybe processed in Cokers and upgraded into petrol, diesel and gas oil, or used directly for asphalt (bitumen).
Conversion
Distillation separates the crude oil into unfinished products. However, the products do not naturally exist in crude in the same proportions as the product mix that consumers demand. The biggest difference is that there is too little petrol and too much heavy oil naturally occurring in crude oil. That is why conversion processes are so important. Their primary purpose is to convert low valued heavy oil into high valued petrol.
All products in the refinery are based on the same building blocks, carbon and hydrogen chains, which are called hydrocarbons. The longer the carbon chain, the heavier the product will be. Converting heavier hydrocarbons to lighter hydrocarbons can be compared to cutting a link on a steel chain to make two smaller chains. This is the function of the "Fluidised Catalytic Crackers (FCCs)", "Cokers" and "Hydrocrackers". In addition to breaking chains, there are times when we want to change the form of the chain or put chains together. This is where the "Catalytic Reformer" and "Alkylation" are necessary. Specialised catalysts are of critical importance in most of these processes.
The FCC is usually the key conversion unit. It uses a catalyst (a material that helps make a chemical reaction go faster, occur at a lower temperature, or control which reactions occur) to convert gas oil into a mix of Liquefied Petroleum Gas (LPG), petrol and diesel. The FCC catalyst promotes the reaction that breaks the heavier chains in the right place to make as much petrol as possible. However, even with the catalyst, the reactions require a lot of heat; therefore the FCC reactor operates at about 530 degrees Celsius.
The heaviest material in the refinery is Vacuum Tower Bottoms (VTB) or "resid." If allowed to cool to room temperature, it would become a solid. In Australia resid is sold into the paving asphalt market or used as a blend component in fuel oil. Resid is too heavy and has too many contaminants to process in the FCC. A Delayed Coker can be used to convert this heavy material into more valuable products. The delayed coker uses high temperature to break the hydrocarbon chains. Delayed coking reactions are less selective than FCC reactions. Delayed coking also produces a relatively low valued petroleum coke as a by-product. (Delayed coking is not used in Australian refineries)
In some refineries, the FCC and Delayed Cokers are supplemented by Hydrocracking. Similar to the FCC, the Hydrocracker uses high temperature and catalyst to get the desired reactions. In Hydrocracking, the catalyst stays in one place and the gas oil passes over the catalyst, whereas in the FCC the catalyst is much finer and moves together with the gas oil. The catalyst compositions differ. In Hydrocracking, the reactions take place at high temperatures in the presence of high concentrations of hydrogen. The Hydrocracker produces products with low sulphur levels. The light liquid product can be sent directly to Catalytic Reforming and the other liquid products can be blended directly into jet fuel and diesel.
The conversion processes that have been discussed up to this point have focused on reducing the length of some hydrocarbon chains. However, there are other hydrocarbon chains that are too short. Butane is produced as a byproduct of other conversion units. The Alkylation Unit (Alky) takes two butanes and combines them into a longer chain using a catalyst.
The last conversion process to be discussed is Catalytic Reforming. The purpose of the reformer is to increase the octane number of petrol blend components and to generate hydrogen for use in the refinery hydrotreaters. The same length carbon chains can have very different octane numbers based on the shape of the chain. Straight chains, or paraffins, have a relatively low octane number, while rings, also called aromatics, have high octane numbers. At high temperatures and in the presence of hydrogen, the catalyst will "reform" paraffins into aromatics, thus the name catalytic reforming. Some of the aromatics produced are sent to petrochemical manufacturers, where they are converted to plastics and fabrics.
Purification
Once crude oil has been through separation and conversion, the resulting products are ready for purification, which is principally sulphur removal. This is done by "Hydrotreating", a process similar to Hydrocracking but without converting heavy molecules into lighter ones. In Hydrotreating, unfinished products are contacted with hydrogen under heat and high pressure in the presence of a catalyst, producing hydrogen sulphide and desulphurised product. The catalyst accelerates the rate at which the sulphur removal reaction occurs. In each case, sulphur removal is essential to meeting product quality specifications and environmental standards. Other units in the refinery remove sulphur, primarily in the form of hydrogen sulphide, through extraction, which is a second method of purification.
Whether through hydrotreatment or extraction, desulphurization produces hydrogen sulphide. Sulphur recovery converts hydrogen sulphide to elemental sulphur and water. The residual sulphur is sold as a refinery byproduct.
End Products
Modern refinery and petrochemical technology can transform crude oil into literally thousands of useful products. From powering our cars and heating our homes, to supplying petrochemical feedstocks for producing plastics and medicines, crude oil is an essential part of our daily lives. It is a key ingredient in making thousands of products that make our lives easier - and in many cases - help us live better and longer lives.

Oil does a lot more than simply provide fuels for our cars and trucks, keep our homes and offices comfortable, and power our industries. From lipstick to aspirin to roller blades, petrochemicals play a vital part. Hera are a few examples of products made from petrochemicals..
- Antiseptics
- Golf Balls
- Aspirin
- House Paint
- Baby Strollers
- Jet Fuel
- Balloons
- Medical
- Equipment
- Cameras
- Motor Oil
- Perfumes
- CD Players
- Photographs
- Clothing
- Roller Blades
- Compact Discs
- Shampoo
- Deodorant
- Sunglasses
- Disposable Nappies
- Telephones
- DVDs
- Tyres
- Toothpaste
- Petrol
- Toys
- Garbage Bags
- Umbrellas
- Glue
- Vitamin Capsules
Reference(s).. ExxonMobil Australia Pty Ltd (www.exxonmobil.com.au)
Other Pages
Part 1..
Introduction to fossil fuels
Part 2..
Oil and Gas exploration
Part 3..
Oil & Gas extraction
Part 4..
Transport & Storage of Crude Oil & Natural Gas
Part 5..
Petroleum Refining Processes
Part 6..
Facts from the Oil Industry
Part 7..
Current Crude Oil prices
Part 8..
Proved Global Oil Reserves
Part 9..
Proved Natural Gas Reserves
Part 10..
The End of the Oil Age?