Catalyst handling
Catalyst processing is the removal of old catalyst and loading of new catalyst into a reactor or processing unit in an oil refinery or chemical plant. This process involves several steps, which vary depending on the type of reactor and the nature of the catalyst
What are catalysts?
A catalyst is a substance that increases the rate of a chemical reaction, but is not usually consumed by the reaction. A catalyst is normally chemically unchanged at the end of the reaction. Many catalysts are complex mixtures of different chemicals.
There are two basic types of catalysis
Homogeneous - The reactants and catalyst are in the same phase, usually in solution. The catalyst is usually recovered from the final product stream through a process such as distillation. The catalyst is reused until the catalytic activity is no longer high enough. Often the raw catalyst material is supplied as a powder and the catalyst must be dissolved in a suitable solvent before the catalyst can be used.
Heterogeneous - The reactants (normally liquid or gaseous) and the catalyst (normally solid) are in different phases. The grains can be spherical, cylindrical or randomly shaped. Many catalysts used in heterogeneous processes are extremely complex. Normally, they consist of a number of main components on an inert carrier material such as alumina or silica. When handled, these solid catalysts can generate airborne dust and thus pose a risk when inhaled.
 Example Of Catalyst Pellets - Image.. Www.Chemspeed.Com
Example Of Catalyst Pellets - Image.. Www.Chemspeed.Com
Catalysts gradually lose their catalytic activity, usually due to structural changes, poisoning, overheating or the deposition of foreign material such as coke. A catalyst is "spent" when it no longer exhibits the necessary activity or specificity required by the user. Depending on the chemical and structural changes in the catalyst during use, the user has a number of options for dealing with the spent catalyst..
- Regeneration and reuse of the material
- Recovery of some or all of the components in the material
- Disposal of the material
Self-heating catalysts are mixtures that can self-heat in large quantities and after extended periods of time (several hours or days) in contact with air and without an energy supply. This can include some fresh and used catalysts. Loading and unloading is normally done under nitrogen to create an inert environment and appropriate packaging and labeling is required. Wet dumping with water is also possible. The self-heating property may be due to the reduced state of the catalyst or to iron sulfide and/or iron sulfide contamination of used catalysts.
The text with the gray background comes from the European Catalyst Manufacturers Association and is taken from the .pdf document below. The document describes everything you need to know, about handling catalysts in reactors.
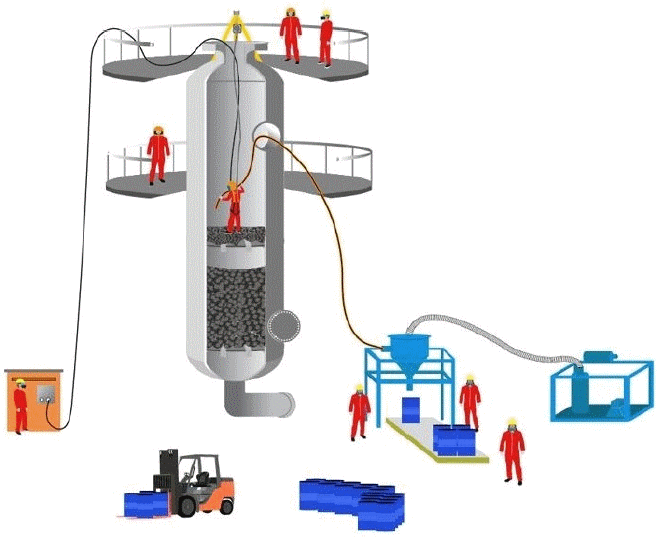
Loading And Unloading Of Catalyst In Reactors / Image.. Breatheairsystem.Com
Related Post(s)
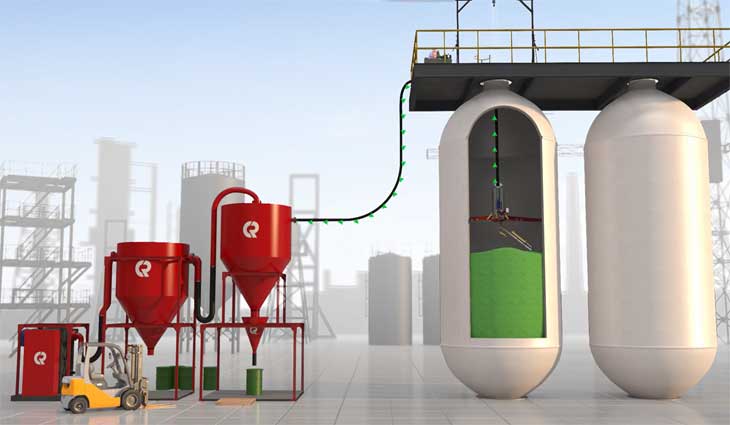
The Catspider is the future of vessel servicing and maintenance. Designed...